Check out today’s Step 1 Qmax Question Challenge.
Know the answer? Post it below! Don’t forget to check back for an update with the correct answer and explanation (we’ll post it in the comments section below).
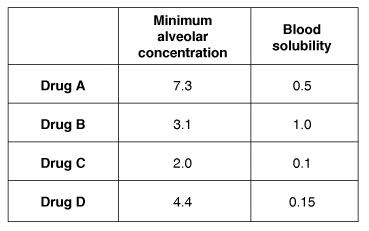 A researcher is examining the different properties of anesthetic gases in a series of experiments. Each gas is determined to have a specific minimum alveolar concentration (MAC) and blood solubility. In the experiments, the MAC of a gas is defined as the end-expiratory concentration at which half of patients will not elicit a motor response to a standardized surgical incision. The inhaled anesthetic drugs A, B, C, and D have the properties indicated in the chart.
A researcher is examining the different properties of anesthetic gases in a series of experiments. Each gas is determined to have a specific minimum alveolar concentration (MAC) and blood solubility. In the experiments, the MAC of a gas is defined as the end-expiratory concentration at which half of patients will not elicit a motor response to a standardized surgical incision. The inhaled anesthetic drugs A, B, C, and D have the properties indicated in the chart.
Which of the following statements best describes the properties of these drugs?
A. Drug A is more potent than drug D
B. Drug B is less soluble in blood than drug C
C. Drug C will have both the highest potency and the most rapid induction
D. Drug D will induce anesthesia more rapidly than drug C
E. Patients treated with drug B will recover more quickly from anesthesia than drug A
———————–
Want to know the ‘bottom line?’ Purchase a USMLE-Rx Subscription and get many more features, more questions, and passages from First Aid, including images, references, and other facts relevant to this question.
This practice question is an actual question from the USMLE-Rx Step 1 Qmax test bank. For more USMLE Step 1 prep, subscribe to our First Aid Step 1 Flash Facts and First Aid Step 1 Express Videos video series. Score the best deal on all three products as a bundle with USMLE-Rx 360 Step 1.




C
C
C
C
I think that the Drug C depicts one of the newer inducing agents used in practice(i.e. SEVOFLURANE/DESFLURANE)
The correct answer is C. The potency of inhaled anesthetics is quantified as the minimum alveolar concentration (MAC). This is the concentration of inhaled gas that is needed to eliminate movement in 50% of patients who are challenged by surgical incision. For potent anesthetics, the MAC will be numerically small, meaning that it is inversely proportional to the anesthetic’s potency. Drug C, which has the smallest MAC value, is thus the most potent. The blood solubility of an anesthetic is the physical property that determines both the speed of induction and time to recovery. Drugs with low blood solubility, such as nitrous oxide, will rapidly induce anesthesia, and patients will recover quickly. In contrast, an anesthetic gas with high blood solubility, such as halothane, will have a longer time to induction and a slower time to recovery. Therefore, drug C, which has the lowest blood solubility, will have the most rapid induction.
A is not correct. The MAC for a drug is inversely related to its potency. Drug A has a larger MAC than drug D and will be less potent than drug D.
B is not correct. The solubility of inhaled anesthetic drugs is described using the blood-gas partition coefficient, which is the ratio of the concentration of the drug in the blood to the concentration of the drug in the gas adjacent to the blood. For drug B, with a solubility of 1.0 (there is an equal concentration of drug B in the blood and in the gas). For drug C, with its solubility value of 0.1, the concentration of drug C in blood is 1/10th that of the concentration in the gas. Therefore drug B is more soluble in blood than drug C.
D is not correct. An anesthetic with greater blood solubility will have a slower induction time. Drug D, which is more soluble in blood than drug C, will therefore induce anesthesia less rapidly than drug C.
E is not correct. Recovery time from anesthesia is based on the blood solubility of a gas. Gases with high blood solubility result in slower recovery than for drugs that have lower blood solubility. Drug B has the highest blood solubility of all those that are listed and thus will have the slowest recovery time of all.