Biostatistics, Study Design, and Epidemiology
This week, we released a new Rx Bricks collection on Biostatistics, Study Design, and Epidemiology. Biostats is an area where medical students aren’t always comfortable, since, unlike anatomy, physiology, and pathology, it deals with areas that heavily involve math and statistics.
However, biostatistics and epidemiology serve as the foundation of evidence-based medicine, and are considered key competencies among practicing physicians. In the era of Covid, these skillsets have become even more critical, whether modeling for the spread of an epidemic or determining the probability of developing effective vaccines.
In this collection, we provide an introduction to the principles and practice of biostatistics, study design, and epidemiology, framed through the lens of public health. The goal is to prepare medical students so that they can be ready to answer questions like:
- Who is impacted by a particular health problem?
- How prevalent is it?
- What is causing the issue?
- Are there ways it can be treated and/or prevented?
Here is a look at what we cover in this new collection, from the fundamentals of statistics to the basics of study design to the basics of epidemiology.
Biostatistics and Study Design
Biostatistics
Fundamentals of Statistics
- Descriptive Statistics and Data Representation
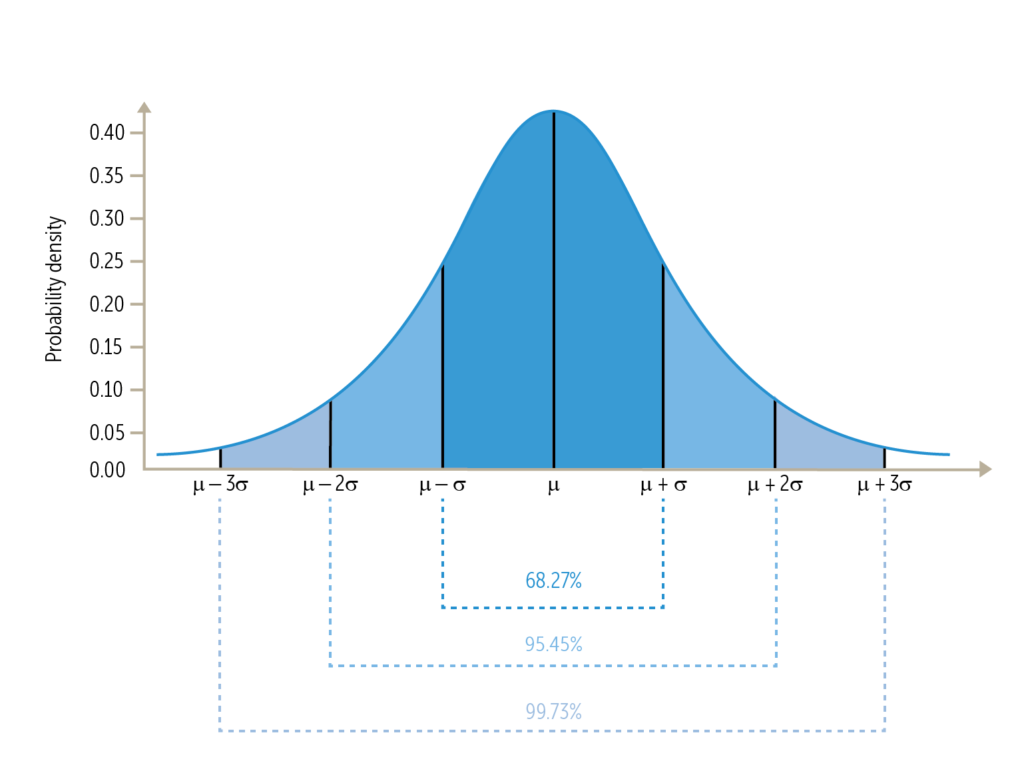
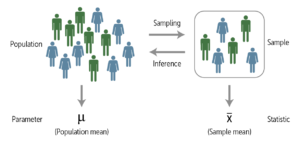
- Inferential Statistics: Sampling Populations
- Statistical Hypothesis Testing: Foundations and Frameworks
Statistical Analysis
- Fundamentals of Statistical Analysis
- Comparative Statistics: Parametric Tests
- Comparative Statistics: Nonparametric Tests
- Comparative Statistics: Correlation and Regression Analysis
Diagnostic Testing
- Diagnostic Test Characteristics
- Applying Tests to Clinical Decisions: Pretest and Posttest Probability
Decision-Making
Reasoning
- The Scientific Method and Research Reasoning
- Clinical Reasoning
Research Studies
Basics of Study Design
- Research Question Creation and Evaluation
- Study Design: Foundations and Frameworks
Observational Studies
- Descriptive Observational Studies
- Analytical Observational Studies
- Analytical Observational Studies: Outcome Measurements
Clinical Studies
- Randomized Controlled Trials: Foundations and Frameworks
- Randomized Controlled Trials: Design and Implementation
- Randomized Controlled Trials: Evaluation and Analysis
Filtered Studies
- Filtered Studies: Meta-analysis and Literature Searches
Elements of Study Design
- Study Design: Qualitative Studies and Mixed Methods Research
- Study Design: Survival Analysis
- Study Design: Sample Size and Power
- Study Design: Bias
- Study Design: Confounding and Effect Modification
Epidemiology and Public Health
Epidemiology
Basics of Epidemiology
- Tools of Epidemiology
- Cost-Effectiveness Analysis
- Clinical Drug Trials